
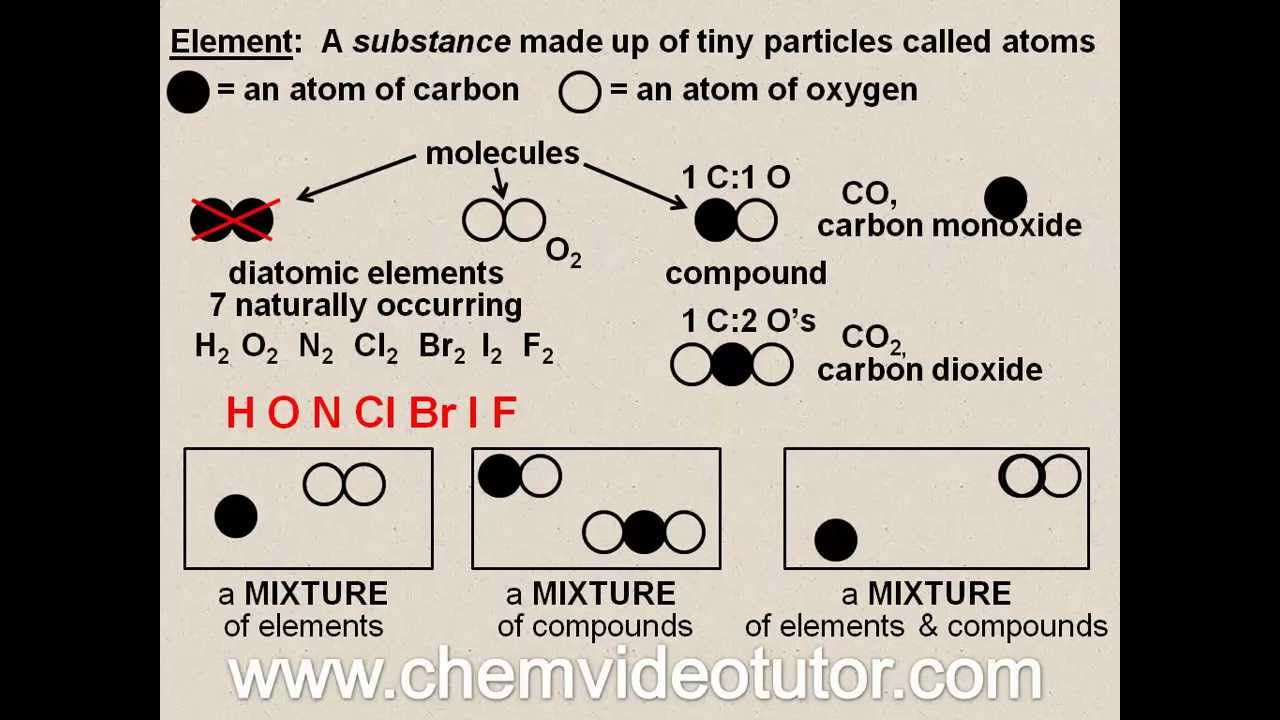
It is interesting to note, just by combinations of a few elements, we can get countless compounds. Hydrogen is flammable and oxygen supports fire while water extinguishes the fire. Hydrogen and oxygen are gases while their compound water is liquid. The properties of elements may or may not resemble its respective compound. We cannot separate the atoms of a constituent particle by any physical methods.Įlements can interact among themselves to form compounds, for example, hydrogen and oxygen react to form water. The atoms of a constituent particle are chemically bonded to each other. In compounds, two or more different atoms combine to form a constituent particle. Examples of compounds are water (H 2O), carbon dioxide (CO2), hydrogen chloride (HCl), ammonia (NH 3), methane (CH 4), glucose (C 6H 12O 6), sodium chloride (NaCl), potassium hydroxide (KOH) etc. When constituent particles of a pure substance are composed of different atoms, it is called a compound. The most abundant elements in the universe are hydrogen and helium. As of 2019, there are 118 elements known to us, and they are listed in the modern periodic table. A representation of atoms (top three) and molecules (bottom three)įor an element, a constituent particle can consist of one or more atoms, but an important point is all the atoms should be the same type. Molecules include hydrogen (H 2), oxygen (O 2), nitrogen (N 2), chlorine (Cl 2) etc. Atoms include sodium (Na), calcium (Ca), silver (Ag), gold (Au), helium (He), neon (Ne). The constituent particles of elements can be atoms or molecules. Some of the examples are elements are sodium, calcium, silver, gold, sulphur, phosphorus, helium, neon, hydrogen, oxygen, nitrogen &c. Pure substances in which constituent particles are composed of the one type of atoms are called elements. The water is represented by these molecules, which are identical and show the same chemical properties.īased on the nature of constituent particles, we can subcategorise pure substances into elements and compounds.

Similarly, the constituent particles of pure water are its molecules (H2O). Each atom in the gold bar is identical to other atoms and they all have the same chemical properties. Constituent particles in the gold bar are its atoms. But they are produced by industries, for example, refining of dore bar (semi-pure gold) by the Wohlwill process, extraction of calcium carbonate from a quarry, electrolysis of sodium chloride to produce hydrogen gas.Ĭonsider a gold bar. Pure substances are normally not found in nature. Examples of pure substances (clockwise from top left: copper dendrites, gold biscuits, calcium carbonate powder, a glass of pure water, and liquid nitrogen.)


 0 kommentar(er)
0 kommentar(er)
